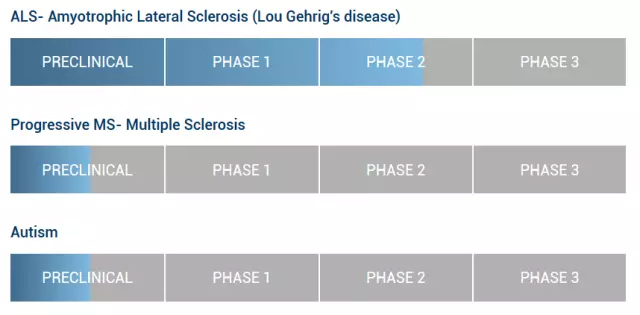
Recently, BrainStorm Cell Therapeutics, a leader in the treatment of adult stem cell technology for neurodegenerative diseases, announced that it will announce NurOwnTM treatment of amyotrophic lateral cord at the annual meeting of the International Society for Cell Therapy (ISCT) in London and the World Conference on Advanced Therapy and Regenerative Medicine. Phase II clinical study data for sclerosing ALS. (It is said that the election was announced in the UK, considering that Hawking’s walking is inconvenient, that is to say, Hawking may arrive at the scene that day, regardless of whether the news is true or not, Hawking’s hearing of this news will be very open.) Brainstorm Cell Therapeutics is a biotechnology company dedicated to the development of adult stem cell therapies for neurological diseases. The company has the right to develop and commercialize NurOwn technology through an exclusive, global licensing agreement with the Tel Aviv University technology transfer company Ramo t. As the core technology of the company, NurOwnTM Cell Therapy can utilize the multi-differentiation potential mesenchymal stem cells in the human body to treat various diseases. NurOwnTM technology is based on a new differentiation protocol that involves inducing human bone marrow mesenchymal stem cells to differentiate into neural cells, forming glial cells and secreting cells, releasing various neurotrophic factors, thereby promoting the growth, survival and differentiation of developing neurons. . The company's core NurOwnTM technology addresses neurodegenerative diseases including amyotrophic lateral sclerosis, multiple sclerosis, and autism. BrainStormCell Therapeutics clinical research pipeline Auto Chemistry Analyzer,Semi Auto Chemistry Analyzer,Veterinary Automatic Chemistry Analyzer,Dry Chemistry Analyzer Guangdong Widinlsa International Co.Ltd , https://www.widinlsamachine.com