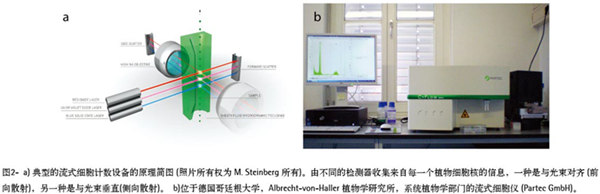
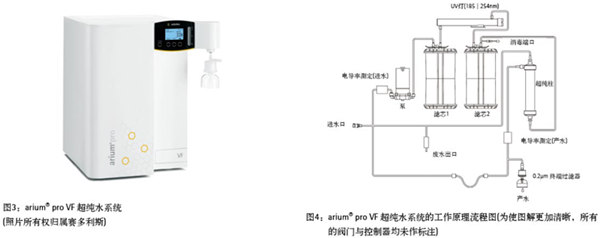
Development of flow cytometry Flow cytometry was originally developed for use in the medical field, but has since evolved into a useful tool in different fields such as cell counting, classification, and biomarker detection [7]. Although the first article on the use of flow cytometry for plant cell nuclear analysis was published in 1973 [8], the analysis of plant DNA using flow cytometry was only leaps and bounds in the late 1980s. Since then, all researchers have been firmly using the technology in the field of plant research. Basically, botanists use flow cytometry to determine the DNA content in the nucleus of a plant cell. See Reference 9 for a detailed description of the principles and applications of flow cytometry. Figure 2a summarizes how a flow cytometer suspends dyed or targeted particles (having a size between 0.2 μm and 150 μm [7]) with a stream of hydrodynamically concentrated liquid through an electronic detection device. In plant research, the nucleus (eg, particles) is first stained with a fluorescent label (eg, DAPI [4',6-diamidino-2-phenylindole] or propidium iodide). Then, each suspended cell nucleus is exposed to a beam of light (usually a laser or UV light), and the path of the light is dispersed and sensed by the detector for analyzing the fluorescence intensity of each individual particle, which is the DNA content of the nucleic acid. The measurement provides information. The combined data of thousands of intact nuclei provides information on the DNA content of individual tissues (see the peaks in Figure 1). The quality and purity of the solution used for sample separation and sample detection is critical because the physical and/or chemical characteristics of up to thousands of particles are analyzed almost simultaneously every second. If a low-quality or unsuitable water supply system is used, the contaminant particles that are not part of the sample will react fluorescently and produce “noiseâ€, which can interfere with the test results and lead to a final error assessment. Therefore, high quality ultrapure water must be used in the experiment. Ultrapure water produced by ultrapure water system achieves ASTM Class I quality An ultrapure water system capable of producing ultra-pure water of ASTM Class I quality is supplied by Sartorius (Göttingen, Germany). Using the ultra-pure water (ArUPH2O) produced by the arium® pro VF water system for flow cytometry analysis, in order to evaluate the effect of ultrapure water on the quality of the analytical results, the authors examined the genital parthenogenesis of angiosperms for production. The reproductive pathway of the seed. This was achieved by comparing the results obtained by testing the samples with ArUPH2O and standard sheath solutions (0.04% sodium azide, 0, 01% detergent), respectively (Partec GmbH). The arium® pro VF system (Figure 3) produces ultrapure water from pretreated drinking water by removing all contaminating particles. The production of ultrapure water requires continuous circulation and a constant water flow rate, which can be achieved by a pump system with pressure control. Conductivity is measured at the inlet and downstream ports, or at the production port. The test arium® pro VF water system described in this article (the older generation has the same technical design as the current new generation arium® pro VF water system, as shown in Figure 3) works with two different filter cartridges. The two filter cartridges are packed with a special activated carbon adsorbent and mixed bed ion exchange resin to produce ultrapure water with a very low total organic activated carbon (TOC) content. In addition, the system has an integrated UV lamp with oxidizing organics and bactericidal effects at 185nm and 254nm respectively. Materials and Methods The seed samples were derived from hexaploid ranunculus plants and collected under free pollination conditions. Seed individuals were crushed in a plastic petri dish with 300 μl of extraction buffer (CyStain UV Precise P, Partec GmbH), then incubated for 10 minutes at room temperature, and then nucleated (30 μl slurry, CellTric®, Partec GmbH) into a 5-mL plastic tube. Thereafter, 1.2 mL of staining buffer (CyStain UV Precise P) was added, and after 60 seconds, the sample was analyzed by a blue fluorescent channel of a flow cytometer (CyFlow Space, Partec GmbH; see Fig. 2b). more content" result A total of 61 individual seeds have rebuilt reproductive pathways. According to the recommended standard procedure, 30 seeds were suspended using sheath fluid and 31 seeds were suspended using ArUPH2O (conductivity: 0.055 μs/cm or 18.2 MΩ × cm resistance compensated to 25 ° C). On average, 2329 nuclei were tested per sample, accounting for 73% of the total counted particles, while the other 27% were expressed as the nucleus or background signal of the G2 phase of the cell cycle. For all seeds analyzed, the average peak position of the embryo and endosperm was calculated based on the average total of the enrichment of each peak. more content" discuss In summary, there are very small differences in the test results obtained, which confirms that the arium system is an excellent and fast and economical alternative for the relative ploidy analysis of plant materials. High quality arium ultrapure water has proven to be very effective in achieving reproducible results in lost cell seed screening techniques. Nonetheless, because additives such as antibiotics and detergents can prevent the formation of biofilms and increase the humidity of containers, pipes, valves, and the inner surface of the flow chamber, reliable operation of the lost cell system can cause long-term or short-term effects. The positive impact, so suppliers generally recommend adding such substances to homemade sheath fluids. In short, ArUPH2O is immediately available for flow cytometry in plant cells. As the loss of cell technology is becoming more and more important in other applications, such as the detection of tumor cells, cell quantitative and morphological differentiation, cell cycle analysis, DNA-RNA content estimation, and apoptosis detection, etc., the overall applicability of ArUPH2O is arium. Ultrapure water creates new opportunities in a range of emerging technologies using flow cytometry. View full story » references 1. Johri, BM Embryology of Angiosperms. Springer-Verlag: Berlin, Ger¬many, 1984. 2. Nogler, GA Gametophytic Apomixis. In: Embryology of Angiosperms; Johri, BM, Ed. Springer-Verlag: Berlin, Germany; pp 475–518. 3. Battaglia, E. The male and female gametophytes of angiosperms—an interpretation. Phytomorphology 1951, 1, 87–116. 4. Asker, SE; Jerling, L. Apomixis in Plants. CRC Press: Boca Raton, FL, 1992. 5. Hojsgaard, DH; MartÃnez, EJ et al. Competition between meiotic and apomictic pathways during ovule and seed development results in clonality. New Phytologist 2013, 197, 336–47 (and supporting informa¬tion in the online version of this article) . 6. Dittrich, W.; Göhde, W. Patent DE 1815352, Flow-through Chamber for Photometers to Measure and Count Particles in a Dispersion Medium; 1968. 7. en.wikipedia.org/wiki/Flow_cytometry. 8. Heller, FO DNS-Bestimmung an Keimwurzeln von Vicia faba L. mit Hilfe der Impulscytophotometrie. (DNA estimation on radicles of Vicia faba L. using pulse cytophotometry; translation of the original German title by Dr. Herbig.) Berichte der Deutschen Botanischen Gesellschaft 1973, 86, 437–41. 9. Dolezel, J. Flow cytometric analysi s of nuclear DNA content in higher plants. Phytochem. Anal. 1991, 2, 143–54. 10. Matzk, F.; Meister, A. et al. An efficient screen for the reproductive pathways using mature seeds of monocots and dicots. Plant J. 2000, 21, 97–108. 11. Paun, O.; Hörandl, E. Evolution of hypervariable microsatellites in apo¬mictic polyploid lineages of Ranunculus carpaticola: directional bias at dinucleotide loci. Genetics 2006, 174, 387–98. Yancheng Rongtai Labware Co.,Ltd , https://www.shtestlab.com