Rabies Vaccine (Vero Cell) for Human Use, Freeze-dried is a preparation of rabies fixed virus CTN-1V inoculated into Vero cell. After cultivation by bioreactor, harvest the virus suspension; further perform the concentration, inactivation, and purification. The vaccine is formulated by adding human albumin, dextran 40 and sucrose through freeze-drying process. The freeze-dried vaccine looks like a white crisp cake which turns into a transparent liquid after reconstitution, without any preservative. Active ingredient: inactivated rabies fixed virus. Excipients: Human albumin, dextran 40, sucrose, sodium chloride, potassium chloride, potassium phosphate monobasic, dibasic sodium phosphate. Diluent for vaccine: sterile water for injection. The specification is 0.5ml/vial after reconstitution. 1 vial (0.5ml) per single human dose. The vaccine titer should be not less than 2.5IU. Rabies Vaccine For Human,Human Freeze Rabies Vaccine,Human Rabies Vaccine Storage,Freeze Rabies Vaccine For Humans Changchun Zhuoyi Biological Co., Ltd , https://www.zypharmaceutical.com
Soft, frosted and flexible kink resistant tubing with unique identification number at intervals of 10 cm
Tamper proof, safe and easy to open port covers to prevent contamination
Manufactured from high quality medical grade PVC sheet
Individually packed in special multi layer pouch and multiples packed in moisture barrier aluminum pouch
Convenient hanger slits and holes allow easy suspension of the bags during collection & storage
Anticoagulant CPD solution preserves whole blood up to 21 days
Anticoagulant CPDA solution preserves whole blood up to 35 days
SAGM solution preserves RBC upto 42 day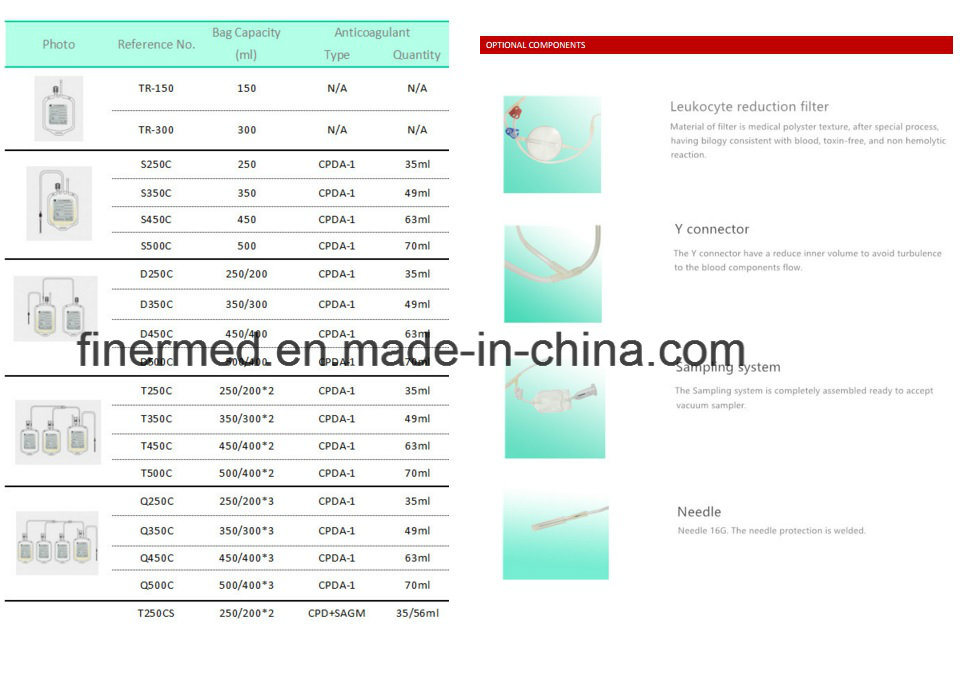
Â
Siliconised ultra thin walled, 16G, sharp Japanese needle assures smooth and atraumatic venipuncture.
Soft, frosted and flexible kink resistant tubing with unique identification number at intervals of 10 cm
Tamper proof, safe and easy to open port covers to prevent contamination
Manufactured from high quality medical grade PVC sheet
Individually packed in special multi layer pouch and multiples packed in moisture barrier aluminum pouch
Convenient hanger slits and holes allow easy suspension of the bags during collection & storage
Anticoagulant CPD solution preserves whole blood up to 21 days
Anticoagulant CPDA solution preserves whole blood up to 35 days
SAGM solution preserves RBC upto 42 day
Â
Model NO.: Blood Bag
Logo Printing: With Logo Printing
Medical Devices Ad. Approval No.: Stxb20140016
Medical Device Regulatory Type: Type 1
Medical Devices Reg./Record No.: Stxb20140016
Trademark: OEM
Transport Package: Box
Specification: universal
Origin: China
HS Code: 9018902000
Model NO.: Blood Bag
Logo Printing: With Logo Printing
Medical Devices Ad. Approval No.: Stxb20140016
Medical Device Regulatory Type: Type 1
Medical Devices Reg./Record No.: Stxb20140016
Trademark: OEM
Transport Package: Box
Specification: universal
Origin: China
HS Code: 9018902000
Siliconised ultra thin walled, 16G, sharp Japanese needle assures smooth and atraumatic venipuncture.