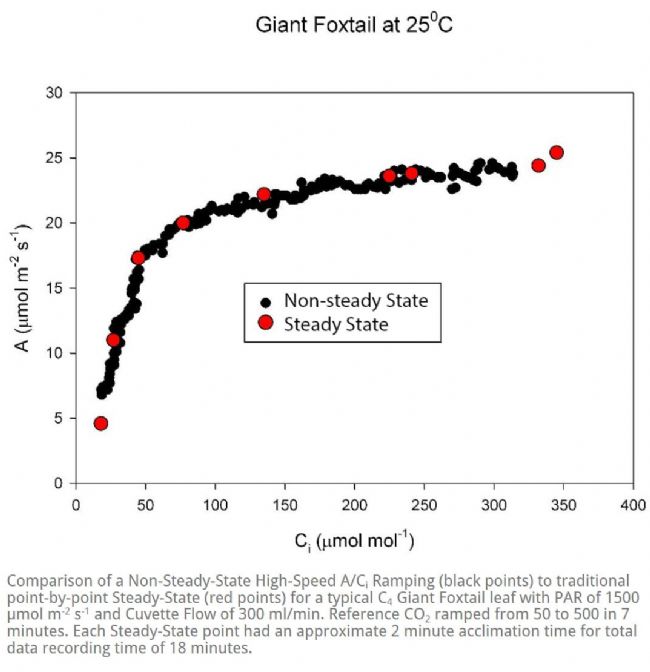
Welcome to the "Lufthansa Technology Group" WeChat public number! Official website: LED lamps generally have excellent fill light efficiency, long life and controllability, and provide narrow-band light. This provides the grower with the means to adjust the physiology of the crop by changing the spectral band and intensity. This requires an in-depth understanding of the effects of light in different bands and different intensities on crop photosynthesis, growth and development. Between red and blue light, the quantum yield of red light is higher than that of blue light, which means crops can use red light more efficiently (Mcree, 1971). However, the collection of McCree data is performed at lower luminous flux density (PPFD) and single band, ignoring the potential interaction between photons of different bands. There is convincing evidence that at higher photon flux densities (PPFD), green light may be more effective than red light (Terashima et al., 2009), while far red light is shorter. The combination of light wavelengths synergistically increases photosynthesis (Zhen and van Iersel, 2017). The effects of different spectra on crop growth and development are not limited to photosynthetic physiology, but may also affect crop morphology and secondary metabolism in a crop-specific manner (Ouzounis et al., 2015). The physiological causes of spectral and crop-specific effects are currently unclear. The traditional method for determining the A/Ci response curve requires the plant to be at a stable light intensity and CO 2 concentration until the plant reaches a stable physiological state, and then the CO 2 concentration is changed for the next gradient. This approach has several drawbacks: Determining a complete A/Ci response curve takes a long time and usually takes at least 30 minutes. Within this time frame, other biological reactions may occur in the plant itself, complicating interpretation (Stinziano et al., 2017). These limitations are addressed by non-steady-state measurements of gas exchange by the latest rapid A/Ci response technology (RACiR) of PPRAS 's CIRAS-3 portable photosynthetic/fluorescence assay system in the United States , which greatly accelerates the measurement of the A/Ci response curve. (Stinziano et al., 2017). RACiR technology quickly adjusts the CO 2 concentration in the chamber and maintains extremely high control accuracy, allowing a complete A/Ci response curve to be measured in minutes. The horticultural physiology laboratory of the University of Georgia in the United States used the CIRAS-3 portable photosynthetic/fluorescence assay system to measure lettuce, and constructed A/Ci response curves under saturated light flux ( 1,000 undefinedmicro; mol/m 2 /s ) of red and blue light, respectively. . Although lettuce leaves have different absorption capacities for red and blue light, the use of saturated PPFDs ensures that this difference in absorbency does not affect the results. 150 photosynthesis data were recorded for each A/Ci response curve, CO 2 in the chamber The concentration control range is 3~950undefinedmicro; mol/mol, and the measurement is completed in 6 minutes. The A/Ci response curve results are shown below. Under red light, the maximum carboxylation efficiency V c,max of lettuce was 37.4 undefinedmicro; mol/ m 2 /s, 27.4 undefinedmicro under blue light ; mol/ m 2 /s was lower than the maximum carboxylation efficiency under red light . This means that red light performs better than blue light in Rubisco's regulated carboxylation activity. The maximum electron transfer rate ( J max ) is 47.3 undefinedmicro; mol/ m 2 /s under blue light , which is also lower than 58.4 undefined micro; mol/ m 2 /s under red light . The use of trifluorophosphate (TPU) also has the same trend (4.16 and 3.30 undefinedmicro under red blue light; mol/m 2 /s ). According to the McCrey action spectrum, red light has a higher CO 2 than blue light. Fixed quantum yield. The difference in quantum yield is related to RubisCO, J max and TPU. The absorption efficiency of chlorophyll to blue light is higher than that of red light. This phenomenon allows red light to reach deeper inside the blade. It also enhances electron transport in deeper cell layers, allowing more cells to participate in leaf carbon dioxide fixation. In addition, some blue light is absorbed by carotenoids and flavonoids, which are less efficient at transferring harvested energy to the reaction center than chlorophyll a and chlorophyll b ( Akimoto et al., 2005 ) . This results in a further decrease in J max blue. Citation: Akimoto, S., M. Yokono, M. Ohmae, I. Yamazaki, A. Tanaka, M. Higuchi, T. Tsuchiya, H. Miyashita and M. Mimuro. 2005. Ultrafast Excitation Relaxation Dynamics of Lutein in Solution and in the Light-Harvesting Complexes Ii Isolated from Arabidopsis thaliana. The Journal of Physical Chemistry B. 109 (25). doi:10.1021/jp050595q McCree, KJ 1971. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agricultural Meteorology. 9 (Supplement C) : 191-216. doi.org/10.1016/0002-1571 ( 71 ) 90022-7 Ouzounis, T., E. Rosenqvist and C.-O. Ottosen. 2015. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience . 50(8): 1128-1135. doi.org/10.21273/ HORTSCI.50.8.1128 Sharkey, TD, CJ Bernacchi, GD Farquhar and Singsaas, EL 2007. Fitting Photosynthetic Carbon Dioxide Response Curves for C3 Leaves. Plant, cell & environment . 30(9):1035-1040. doi:10.1111/j.1365-3040.2007. 01710.x Stinziano, JR, Morgan, PB, Lynch, DJ, Saathoff, AJ, McDermitt, DK, and Hanson, DT 2017. The Rapid A–Ci Response: Photosynthesis in the Phenomic Era. Plant Cell & Environment , 40: 1256– 1262. Doi: 10.1111/pce.12911. Van Iersel, MW and D. Gianino. 2017. An Adaptive Control Approach for Light-Emitting Diode Lights Can Reduce the Energy Costs of Supplemental Lighting in Greenhouses. HortScience . 52(1): 72-77. doi: 10.21273/HORTSCI11385-16 Disposable Latex Gloves,Disposable Vinyl Gloves,Nitrile Glove,Latex Glove ZHANGJIAGANG DINSHENGLIN TRADING CO.,LTD , https://www.dslhouse.com Many greenhouses use supplemental lighting to extend the growth cycle and increase crop yield. However, the cost of lighting electricity is high. It is estimated that the cost of electricity associated with supplemental lighting may account for 30% of operating costs (van Iersel & Gianino, 2017). With the rapid development of technology, growers can now choose light-emitting diodes (LEDs) for fill-in lighting to reduce the high electricity bill associated with supplemental lighting.
Many greenhouses use supplemental lighting to extend the growth cycle and increase crop yield. However, the cost of lighting electricity is high. It is estimated that the cost of electricity associated with supplemental lighting may account for 30% of operating costs (van Iersel & Gianino, 2017). With the rapid development of technology, growers can now choose light-emitting diodes (LEDs) for fill-in lighting to reduce the high electricity bill associated with supplemental lighting. 
Many commercially available growth lamps use red and blue LEDs because of their high efficacy. Red and blue light are also the absorption peaks in the photosynthesis of plant photosynthesis, and are also the most efficient in plant photosynthetic CO 2 fixation in the whole spectrum (McCree, 1971). 
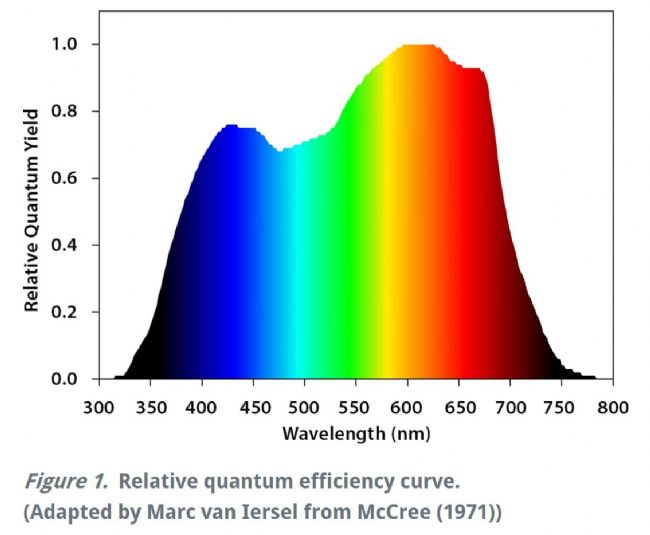 In order to explore the physiological mechanism of red-blue light reaction, Dr. Marc van Iersel from the UGA Horticulture Physiology Lab of the United States constructed a rapid A/Ci response curve to quantify the photosynthetic characteristics of lettuce plants under both red and blue LED illumination. .
In order to explore the physiological mechanism of red-blue light reaction, Dr. Marc van Iersel from the UGA Horticulture Physiology Lab of the United States constructed a rapid A/Ci response curve to quantify the photosynthetic characteristics of lettuce plants under both red and blue LED illumination. . 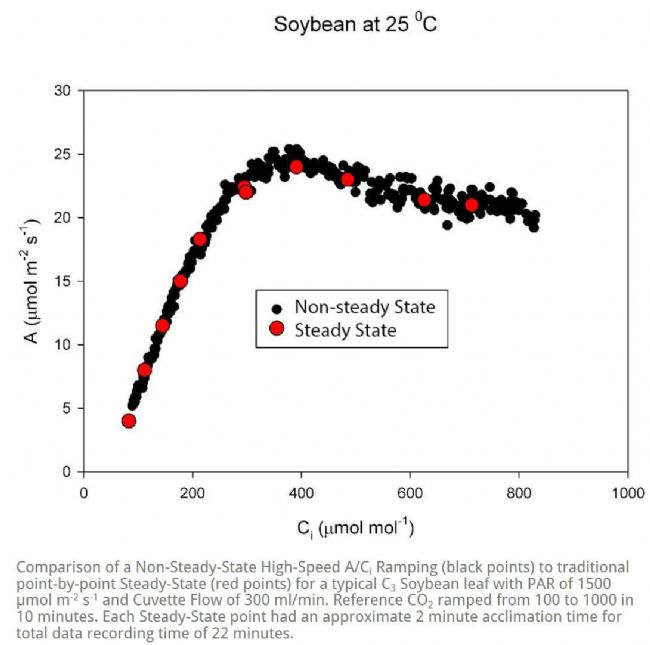
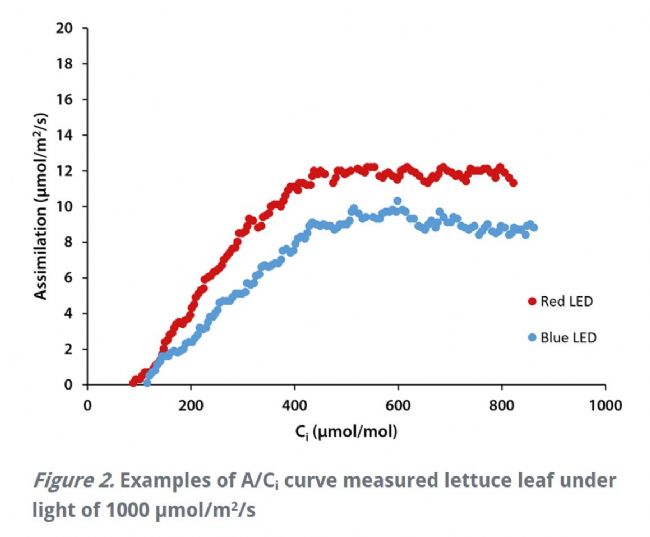
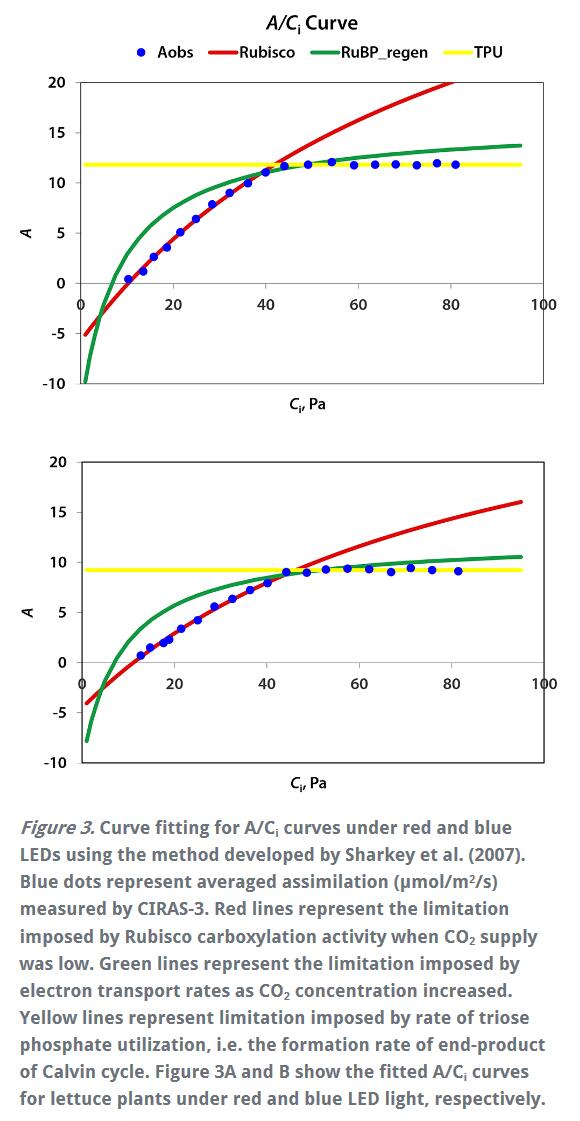
 Also, some Calvin cycle enzymes require light to activate, and deep penetration of light into the leaves may therefore help activate Calvin's circulating enzymes in more cells. Enhanced electron transport and Calvin cycle activity in deeper cell layers can increase the total photosynthetic capacity of the leaves. This is consistent with the results of the fast A/Ci curve of the Laboratory of Horticultural Physiology at the University of Georgia, USA .
Also, some Calvin cycle enzymes require light to activate, and deep penetration of light into the leaves may therefore help activate Calvin's circulating enzymes in more cells. Enhanced electron transport and Calvin cycle activity in deeper cell layers can increase the total photosynthetic capacity of the leaves. This is consistent with the results of the fast A/Ci curve of the Laboratory of Horticultural Physiology at the University of Georgia, USA .
Different spectra have a great influence on the maximum carboxylation efficiency and electron transport rate of plant photosynthesis