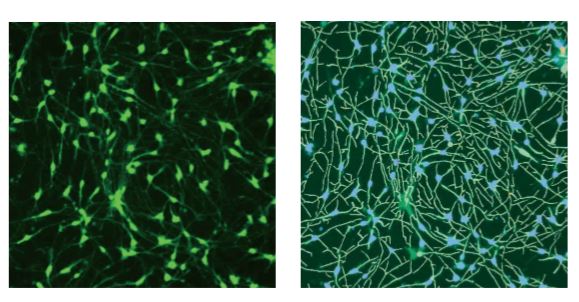
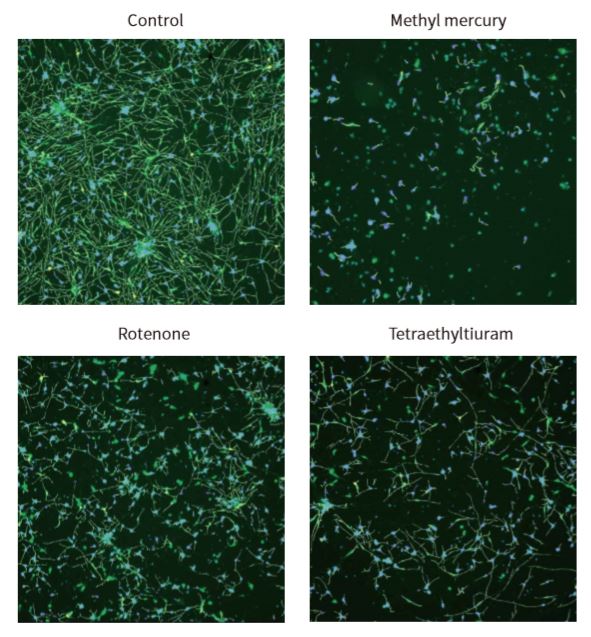
Introduction The growing concern about untested chemicals in the environment has created an urgent need to develop reliable and effective screening tools to identify chemicals that may affect human health, particularly the development of the nervous system. We evaluated a method for measuring neurite outgrowth by screening and characterizing the activity of selected compounds to assess whether they might adversely affect the developing nervous system. This assay was chosen because of its relevance in models of critical processes in the development of the nervous system, particularly as neurons extend their neurites to form a complete neural network. Destruction of this process may have adverse effects on humans and rodents, so studies have shown that immature, developing and mature neurites are targets for chemical toxicity. Although neurite outgrowth assays are primarily used to assess developmental neurotoxicity, they can also be used to assess neurodegeneration by detecting neurite contractions. In addition, this assay may also be associated with assessing neural plasticity in adult neurons. For screening assays, total neurite outgrowth is often the most common metric in indicator reporting. Automated imaging also enables multi-parameter evaluation of other features, such as total number of branches and total number of processes, to assess the different ways in which compounds can inhibit neurite outgrowth. material Identification of compounds with neurotoxicity using iPS cell-based neurite growth assay Human iPSC-derived neurons (i.e., iCell neurons) cells provided by the International Committee on Cellular Dynamics (CDI) are a mixture of post-mitotic GABA reactive groups and glutamatergic neurons. The neurons used in these studies have been cultured by the manufacturer into fully differentiated and purified cell populations and have formed a network of neurites positive for the neuronal markers β-III tubulin and MAP28. Cells were frozen when received, then thawed and plated according to the CDI recommended protocol. Cells were seeded in poly-d-lysine pre-coated 384-well plates and treated with 3.3 μg/mL laminin. Prior to compound treatment, 7,500 cells were seeded per well and grown in iCell Neurons medium for 48 hours. The neurite network in these cells usually begins to form about 24 hours after inoculation and begins to add complexity when cultured to 10-12 days. At 48 hours after inoculation, we evaluated neurite outgrowth, and at the same time, neuronal atrophy may occur. Compounds were tested in duplicate in six concentration ranges (0.3, 1.0, 3.0, 10.0, 30.0 and 100 [mu]M). Multiple DMSO controls (n=16) and untreated controls (n=16) were included in each plate. Solvent effects in the assay were evaluated using up to 0.3% DMSO. The cells were exposed to the compound for 72 hours at 37 ° C and 5% CO 2 . Next, the medium was removed, and the cells were fixed with 4% paraformaldehyde for 2 hours. Permeabilization was carried out in PBS containing 1% fetal bovine serum and 0.01% saponin. Subsequently, cells were incubated with anti-β-tubulin III (TUJ-1) antibody (1:100 dilution) and 2 μg/mL Hoechst-conjugated AF488 dye-conjugated mouse anti-human antibody for 3 hours. Beta-tubulin is used as a marker for neurite outgrowth and is also used for the counting of intact neuronal cell bodies. After incubation, the staining solution was replaced with phosphate buffered saline (PBS). Alternatively, viable cells can be used for neurotoxicity assays, stained with calcein AM and Hoechst dyes (0.5 [mu]M and 2 [mu]M, respectively) for 30 minutes. Detailed information on seeding density optimization and protocols for 384-well format determination are described in detail in Sirenko et al. Images of each well were taken using a 10x PlanFluor objective of the ImageXpressNano automated imaging system. A 10x image was captured at a single site in each well of a 384-well plate. The 10x objective provides sufficient resolution to distinguish the neurite network and subcellular structure of a large number of cells (500-1,000) in each image, covering approximately 1/4 of the total pore area of ​​the table. After image acquisition, all image analysis was done using CellReporterXpressTM automated image acquisition and analysis software, which included image processing application modules for neurite outgrowth and viability assessment. As an example of image processing, Figure 1 shows a magnified typical image from a neurite image and a corresponding analysis layer. Figure 2 shows images of DMSO-treated neurons and compound-treated neurons with a software-tracked overlay on their respective structures. Figure 1. ß-tubulin (green) stained images and software analysis traces of control cells. iCell neurons were plated for 5 days, fixed, and stained with AF 488-conjugated anti-beta-tubulin (TUJ-1) antibody (1:100). The image was taken by the ImageXpress Nano system using a 10x Plan Fluor objective and a FITC channel. The image is processed using the Neurolite Tracing analysis algorithm in the CellReporterXpress software. The analysis layer on the right shows cell growth (green) and cell body (blue) Figure 2. Composite image of ß-tubulin (green) and Hoechst (blue) showing the analytical trajectories of control cells and cells treated with selected compounds. iCell neurons were plated for 48 hours, treated with compounds for 72 hours, then fixed and combined with Hoechst (2 μM) and anti-TUJ-1 antibody (1:100) with AF 488 fluorescence. Images were taken by the ImageXpress Nano system using a 10x Plan Fluor objective for DAPI and FITC channels. The image is processed using the Neurolite Tracing analysis algorithm in the CellReporterXpress software. Destructed neurite networks and cell death in neurons treated with the indicated compounds were observed. Get the full content click the button below Spring Safety Vest,Safety Safety,Shirt Hi Vis Jacket Ningbo Staneex Imp. & Exp. Co., Ltd. , https://www.staneex.com
• ImageXpressNano automatic imaging system with CellReporterXpress automatic image acquisition and analysis software (MolecularDevices)
• iCell® Neuron (CellularDynamicsInternational)
• Poly-d-lysine pre-coated with 384-well plates (CorningLifeSciences)
• Laminin (Sigma-Aldrich)
• Paraformaldehyde (Sigma-Aldrich)
• Fetal bovine serum (Sigma-Aldrich)
• β-tubulin III (TUJ-1) (BD Biosciences)
•Hoescht (ThermoFisherScientific)
• Anti-β-tubulin antibody (BDBiosciences)
• CalceinAM (ThermoFisherScientifiic)