New systemic sclerosis drug is eligible for FDA fast track today
March 20, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, Boehringer Ingelheim announced that the FDA has granted the company the nintedanib Fast Track designation. Nintedanib is a new drug in the treatment of patients with systemic sclerosis with associated interstitial lung disease (SSc-ILD). Fast Track Qualification is used to help treat serious illnesses and to fill new therapies for unmet medical needs. This qualification is based on the expected efficacy and safety data for the study of new drug applications for nintedanib for SSc-ILD and phase 3 trials for SENSCIS.

Systemic sclerosis, also known as scleroderma, is a rare disease that causes hardening and scarring of connective tissue in multiple organs in the human body. Most patients develop a certain degree of lung scar tissue, the interstitial lung disease, in the lungs. This is usually the leading cause of death in patients with systemic sclerosis. There are currently no FDA-approved drugs to treat the disease, and the drugs under investigation in clinical trials are very rare.
Nintedanib is a small molecule tyrosine kinase inhibitor developed by Boehringer Ingelheim, which has been approved for the treatment of rare lung diseases called idiopathic pulmonary fibrosis (IPF). Nintedanib is able to inhibit the activity of receptor tyrosine kinases such as PDGF, FGF, and VEGF. These receptor tyrosine kinases are thought to be the pathological processes of vector IPF. Nintedanib is effective in reducing the rate of decline in lung function in patients with IPF each year. Because of the similarities between SSc-ILD and IPF in the formation of scar tissue or fibrosis in the lungs, Boehringer Ingelheim decided to test the efficacy of nintedanib in the treatment of SSc-ILD.
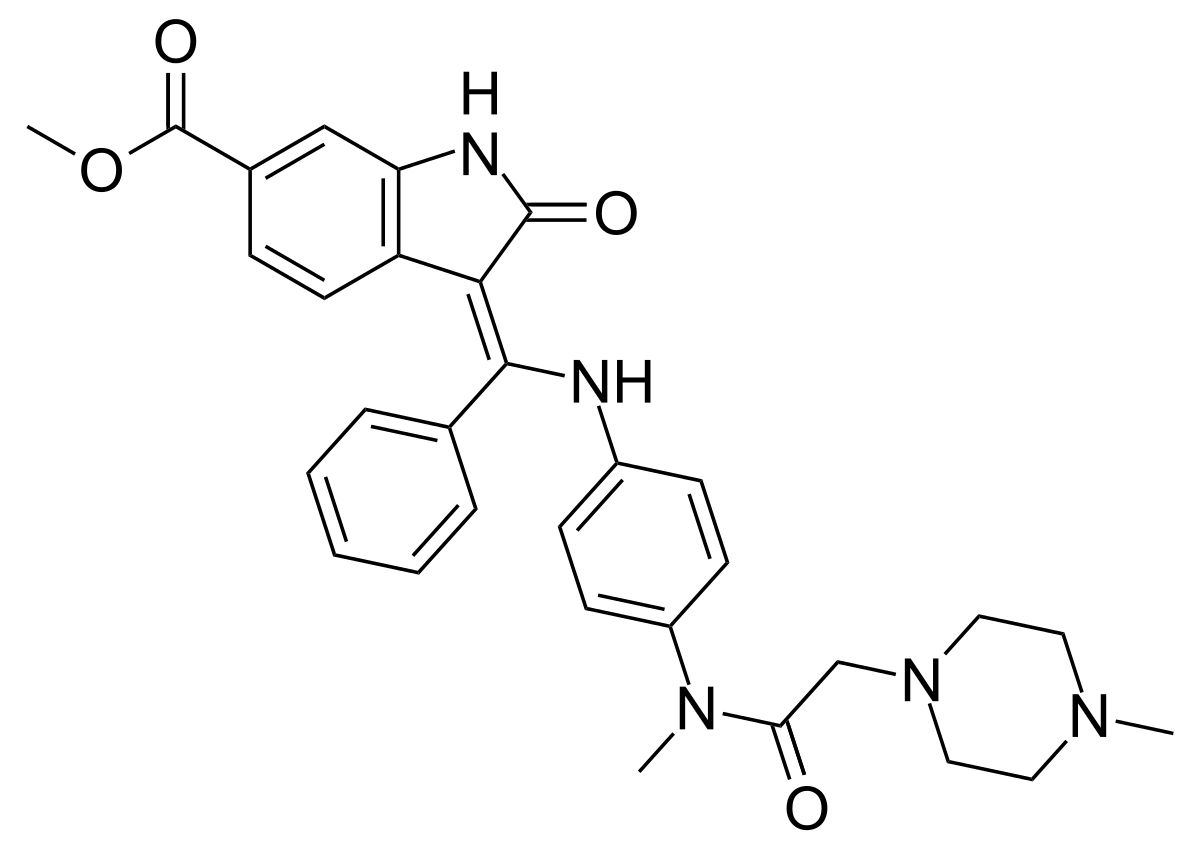
â–²Nintedanib's molecular structure (Source: By Ed (Edgar181) (Own work) [Public domain], via Wikimedia Commons)
In a randomized, double-blind, placebo-controlled, global clinical phase 3 trial called SENSCIS, patients with SSc-ILD will receive nintedanib for 52 to 100 weeks. The primary endpoint of the trial was annual changes in forced vital capacity, an indicator of the progression of lung disease. The patient recruitment process for this trial has now been successfully completed, and more than 520 patients enrolled in 32 countries will participate in this critical clinical trial.
“Fast channel eligibility is a welcome step forward for us to improve our healthcare care for SSc-ILD patients,†said Dr. Christopher Corsico, Bolinger Ingelheim’s Chief Medical Officer. “It’s critical to fill the serious unmet medical needs of patients with these diseases. , We look forward to continuing to work with the FDA to further advance the development of this potential therapy."
Reference materials:
[1] FDA grants Fast Track designation to nintedanib for the treatment of systemic sclerosis with associated interstitial lung disease
[2] Ofev (Nintedanib): First Tyrosine Kinase Inhibitor Approved for the Treatment of Patients with Idiopathic Pulmonary Fibrosis
1.uncut sheet for urine test low price
2.uncut sheet for urine test high quality
3.uncut sheet for urine test rapidly
4.uncut sheet for urine test OEM design welcome
The benefit to purchase rapid uncut sheet for urine test strip :
To help you saving the shipping cost
To help you being the manufacture
To help you saving the import tax as the materials
Our factory can supply more than 30 kinds of rapid test sheets
Package: aluminum foil package
Advantage: vacuum package can protect the effect of moisture
Uncut Sheet,Uncut Sheet Strips,Rapid Uncut Sheet,Uncut Sheet Urine Strips
Changchun LYZ Technology Co., Ltd , https://www.lyzstrips.com