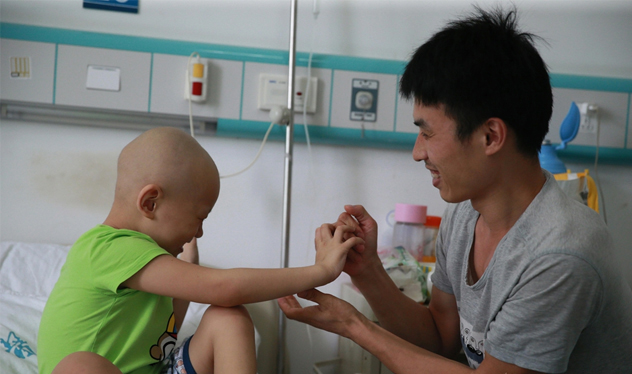
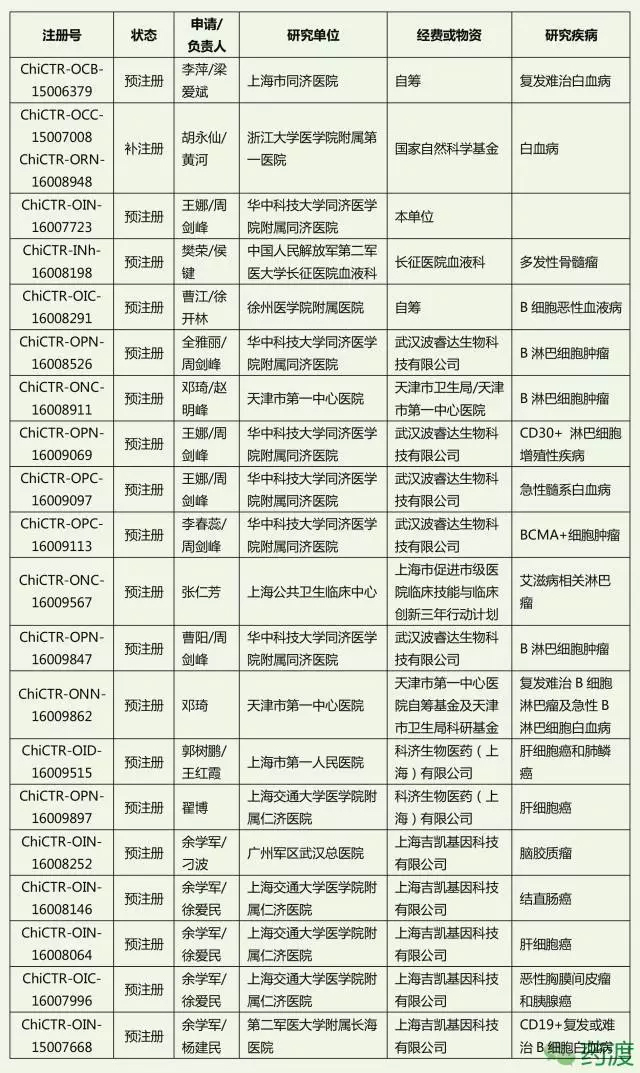
Recently, due to clinical trials that caused two patients with acute lymphoblastic leukemia to die during treatment, Juno announced that it would abandon its CAR-T product (CD28zCAR) JCAR015 with CD28 as a costimulatory domain, and instead focus on developing CD19. Target, CAR-T product (4-1BBzCAR) JCAR017 and JCAR014 with 4-1BB as costimulatory domain. On the other hand, Kite is also the CD28 CAR-TKTE-C19 due to eye-catching clinical data, as of March 10, Kite has completed a total financing of 409 million US dollars. The same technology, different results, inevitably makes people feel. At present, domestic reports on the application of CAR-T technology have also increased. Not only is there an international cooperation model similar to that of the First Affiliated Hospital of Zhejiang University, but also an application report of independent research. The affiliated hospital of Xuzhou Medical College successfully used the modified version of CAR-T cell targeting CD19 to treat acute lymphoblastic leukemia (ALL) using scFv sequence. The pediatrics of the Second Hospital of Hebei Medical University used CAR-T to treat acute lymphoblastic leukemia. Successful case, Beijing Kate Medical Technology Co., Ltd. and Beijing Children's Hospital Department of Hematology use CD19 CAR-T cell therapy gene hematopoietic stem cell transplantation (alloHSCT) recurrence of acute B lymphocytic leukemia, Renji Hospital Shanghai Cancer Institute adopts CAR -T cell infusion therapy for malignant glioma, infusion of CAR-T cells in Hefei Binhu Hospital for the treatment of relapsed and refractory B cell lymphoma, the first case of Shenzhen Second Hospital CAR-T cell for gastric lymphoma, southwest Chongqing The application of CAR-T for leukemia in the treatment of CD19-positive acute lymphoblastic leukemia (ALL) and B-cell malignant tumors in the Department of Hematology, Hospital Biotherapy Center, more such as: Xiangya Third Hospital, Wuhan Tongji Hospital Guyuan District, Nankai University & Tianjin People's Hospital, Second Affiliated Hospital of Anhui Medical University, People's Liberation Army General Hospital, Union Hospital of Fujian Medical University, and so many hospitals and research institutions on technology research and application of CAR-T have achieved some exciting results. A total of 21 clinical trials were conducted from the National Clinical Trial Registry with the words "chimeric antigen" and "CAR-T". The details are as follows: In addition, the related patents 220 and 94 were searched for by “chimeric antigen†and “CAR-T†respectively. By analyzing the content of the applicant and analyzing the patent content of the invention, the patent information authorized by the relevant domestic research is as follows: : Dual chimeric antigen receptor-modified T lymphocytes and preparation method thereof; the invention constructs a low affinity chimeric antigen receptor (CAR-L) and a high affinity chimeric antigen receptor (CAR-H), respectively, to recognize two tumors Related antigens, and contain CD3ζ sequence and costimulatory molecule signal sequence (CM) sequence respectively; Shenzhen Yinguan Biotechnology Co., Ltd. Best Eyelash Curler,Eyelash Curler Price,Good Eyelash Curler,Cheap Eyelash Curler Shenzhen Jie Zhong Lian Investment Co., Ltd. , https://www.szmeizonscares.com