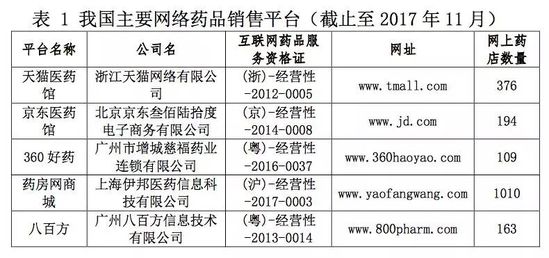
A few days ago, the Southern Pharmaceutical Economic Research won the bid for the “Network Drug Information and Transaction Monitoring System Construction Project†by the State Food and Drug Administration at a price of 1.98 million yuan. For the reason for the bidding of the project, it was mentioned in the published Consultation Document on Network Drug Information and Transaction Detection System Project (hereinafter referred to as the consultation document), because “network drug transactions are concealed, virtual and trans-regional. The traditional methods of supervision and inspection have been difficult to adapt to the current needs of the new regulatory situation. In view of this, it is urgent to show the unified monitoring work of the whole network, and use information technology to improve the efficiency of supervision and ensure the quality and safety of drugs." The purpose of the project is to "use information technology and means to complete the entire network search for drug information and trading websites suspected of violations of laws and regulations, and further strengthen the supervision of online drug information release and drug sales." Who is monitoring? How to monitor? The online drug information and transaction monitoring conducted by the South is not only for online sales of prescription drugs, but also for illegal drug transactions such as overseas drug purchases and drug sales that have not been approved by the Chinese medicine. The monitored network drug sales platforms include: Tmall Medicine Museum, Jingdong Medicine Museum, 360 Good Medicine, Pharmacy Network Mall, and 800 square meters. The Consultation Document also mentioned that after the project was launched, the PC-side website was first included in the monitoring scope, and then mobile social media and App tools such as Weibo and WeChat were gradually included in the monitoring scope. So how do you monitor these companies? Five directions are proposed in the Consultation Document: 1. For websites with drug information and transaction service qualifications, it is required to provide the necessary technical data back-end interface, through product information capture comparison, image technology analysis comparison, forensic packaging and push to the food and drug supervision department of the website registration site; 2. For websites that do not have Internet drug information and transaction service qualifications, they regularly search for violations of laws and regulations, conduct evidence collection in a timely manner, and provide regular feedback. 3. The monitoring system is connected to the third-party notary department, and the notarized forensic data ensures the legality and enforceability of the results of the evidence collection. 4. The monitoring system is connected with the provincial food and drug regulatory authorities to realize real-time push of monitoring data and feedback on law enforcement results. 5. Interconnect with the national food and drug regulatory data sharing platform to achieve data interconnection. The Consultation Document also puts forward requirements for the monitoring system. Among them, the basic information management subsystem requires the functions of drug enterprise information database management, drug information database management, black and white list management, and regulation management; Provides collection management in the monitoring management subsystem (requires 7*24 unattended information collection, with periodic batch collection, breakpoint resuming, etc.), comparison management, storage management, forensic management, push management and other functions. How to punish violations? Industry insiders told Yibang Power Network that after the South China won the bid, it was only a regulatory department, with only regulatory power and no enforcement power. In the "Consultation Document" mentioned: "Automatic screenshots, automatic analysis, automatic forensics packaging for the discovery of violations of laws and regulations. Record relevant forensic process information and evidence collection results information." "Automatically push to the food and drug supervision of the website business subject jurisdiction department." The final punishment power is still in the hands of local administrative agencies. However, the industry insiders also told Yibang Power Network that there is no “appropriate†punishment for illegal drug sales in China, and most of the cases will be sold directly to the public by means of postal sales or internet transactions. The prescription drug punishment method is used to deal with it: “order the correction, give the warning, and impose a fine of not more than two times the value of the drug, but the maximum is not more than 30,000 yuanâ€. Network sales platform, afraid? A number of industry insiders confirmed to Yibang Power Network that as early as two years ago, the South had revealed that it had been commissioned by the State Food and Drug Administration to conduct Internet drug sales behavior monitoring. At present, no results have been monitored. As the "old drivers" of the medical and telecommunications business, each platform has countermeasures under the policy. According to industry insiders, only the platform sales of prescription drugs, each business has a different way of coping, the most common is to hear the inspection of the wind, not only choose the off-the-shelf product, or through the Internet drug information display qualification certificate for prescription drug display. The industry insiders have different views on the upcoming drug monitoring system in the South. Some industry insiders believe that the significance of the monitoring system is not great, and the food and drug supervision bureaus around the country have different regulatory attitudes toward medical e-commerce. Some people in the industry directly pointed out that the main purpose of establishing the system is to help the CFDA to be exempted. The responsibility for related issues will be directly borne by the local food and drug administration and the monitoring system. Some people think that it will have an impact on the industry. Since last year, although it seems that the "threshold" of medical e-commerce is getting lower and lower, ABC certificates have been canceled, but the regulatory system has gradually been established. Billion State Power Network learned that the "Network Drug Administration Supervision and Management Measures (Draft for Comment)" issued by the CFDA last November clearly stated: "The network drug sellers are drug retail chain enterprises, and they are not allowed to sell prescription drugs through the Internet. "Management of required drugs" and "Sites that sell drugs to individual consumers may not publish prescription drug information through the Internet." Even in regular hospitals and under the guidance of a doctor, patients may experience discomfort or even death. So who is responsible for the problem with online prescription drugs? Medical e-commerce not only involves industrial and commercial law enforcement departments, but also drug regulatory authorities. An industry insider who has been engaged in policy analysis for the pharmaceutical industry for many years told Yibang Power Network: Suppose a Beijing patient buys a drug at a Shanghai-registered online pharmacy, which is distributed by a third-party logistics company in Guangzhou. Finally, the patient has a problem with taking the drug. Who is responsible for this? Will the Southern Monitoring System find a solution to the above phenomenon? Tetanus Vaccine,Hepatitis B Vaccine For Adults,Tetanus Booster,Td Vaccine FOSHAN PHARMA CO., LTD. , https://www.pharmainjection.com