Sweeteners are Food Additives that impart sweetness to soft drinks. Sweeteners can be divided into nutritive sweeteners and non-nutritive sweeteners according to their nutritional value. According to its sweetness, it can be divided into low-sweetness sweet agent and high-sweetness sweet I flavor agent. According to its source can be divided into natural sweeteners and synthetic sweeteners.High safety, good taste, high stability, good water solubility, and reasonable price.Sweeteners,Preservatives,Thickener, Acidity regulator,Food Additives. Sweeteners,Preservatives,Thickener, Acidity regulator,Food Additives Xi'an Henrikang Biotech Co.,Ltd , https://www.xianhenrikang.com
Keywords: AZD-3759, AZD-3759 powder, AZD-3759 raw powder, AZD-3759 price, AZD-3759 supplier, AZD-3759 dosage, AZD-3759 benefit
Common Name   AZD-3759
CAS Number:1626387-80-1
Purity: 98 %
Appearance: Off-white solid
Solubility: Soluble in 0.1N HCl(aq) and DMSO
Molecular Formula:C22H23ClFN5O3
Molecular Weight:459.901
Density:1.4±0.1 g/cm3
Boiling Point:571.4±50.0 °C at 760 mmHg
Melting Point:192.4 °C, 193.3 °CÂ
Flash Point:299.3±30.1 °C
Storage  Â
Powder: -20°C for 3 years;  4°C for 2 years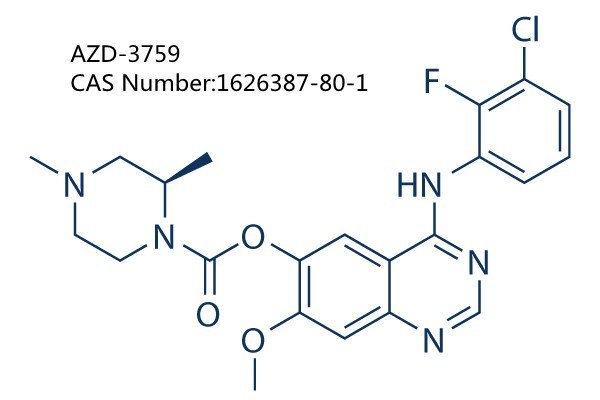
AZD3759 is a potent, oral available, CNS-penetrant EGFR inhibitor with IC50 of 0.3 nM, 0.2 nM, and 0.2 nM for EGFR (WT), EGFR (L858R), and EGFR (exon 19Del), respectively. AZD3759 binds to and inhibits the activity of EGFR as well as certain mutant forms of EGFR. This prevents EGFR-mediated signaling, and may lead to both induction of cell death and inhibition of tumor growth in EGFR-overexpressing cells.
Non-small-cell lung cancer patients with activating mutations in epidermal growth receptor (EGFR) respond to EGFR tyrosine kinase inhibitor (TKI) treatment. Nevertheless, patients often develop central nervous system (CNS) metastases during treatment, even when their extracranial tumors are still under control. In the absence of effective options, much higher doses of EGFR TKIs have been attempted clinically, with the goal of achieving high enough drug concentrations within the CNS. Although limited tumor responses have been observed with this approach, the toxicities outside the CNS have been too high to tolerate. We report the discovery and early clinical development of AZD3759, a selective EGFR inhibitor that can fully penetrate the blood-brain barrier (BBB), with equal free concentrations in the blood, cerebrospinal fluid, and brain tissue. Treatment with AZD3759 causes tumor regression in subcutaneous xenograft, leptomeningeal metastasis (LM), and brain metastasis (BM) lung cancer models and prevents the development of BM in nude mice. An early clinical study in patients with BM and LM treated with AZD3759 confirms its BBB-penetrant properties and antitumor activities. Our data demonstrate the potential of AZD3759 for the treatment of BM and LM and support its further clinical evaluation in larger trials.Â
Â
AZD-3759 Raw Powder Purity 99% oral CNS-penetrant EGFR inhibitorÂ
Keywords: AZD-3759, AZD-3759 powder, AZD-3759 raw powder, AZD-3759 price, AZD-3759 supplier, AZD-3759 dosage, AZD-3759 benefit
Common Name   AZD-3759
CAS Number:1626387-80-1
Purity: 98 %
Appearance: Off-white solid
Solubility: Soluble in 0.1N HCl(aq) and DMSO
Molecular Formula:C22H23ClFN5O3
Molecular Weight:459.901
Density:1.4±0.1 g/cm3
Boiling Point:571.4±50.0 °C at 760 mmHg
Melting Point:192.4 °C, 193.3 °CÂ
Flash Point:299.3±30.1 °C
Storage  Â
Powder: -20°C for 3 years;  4°C for 2 years
AZD3759 is a potent, oral available, CNS-penetrant EGFR inhibitor with IC50 of 0.3 nM, 0.2 nM, and 0.2 nM for EGFR (WT), EGFR (L858R), and EGFR (exon 19Del), respectively. AZD3759 binds to and inhibits the activity of EGFR as well as certain mutant forms of EGFR. This prevents EGFR-mediated signaling, and may lead to both induction of cell death and inhibition of tumor growth in EGFR-overexpressing cells.
Non-small-cell lung cancer patients with activating mutations in epidermal growth receptor (EGFR) respond to EGFR tyrosine kinase inhibitor (TKI) treatment. Nevertheless, patients often develop central nervous system (CNS) metastases during treatment, even when their extracranial tumors are still under control. In the absence of effective options, much higher doses of EGFR TKIs have been attempted clinically, with the goal of achieving high enough drug concentrations within the CNS. Although limited tumor responses have been observed with this approach, the toxicities outside the CNS have been too high to tolerate. We report the discovery and early clinical development of AZD3759, a selective EGFR inhibitor that can fully penetrate the blood-brain barrier (BBB), with equal free concentrations in the blood, cerebrospinal fluid, and brain tissue. Treatment with AZD3759 causes tumor regression in subcutaneous xenograft, leptomeningeal metastasis (LM), and brain metastasis (BM) lung cancer models and prevents the development of BM in nude mice. An early clinical study in patients with BM and LM treated with AZD3759 confirms its BBB-penetrant properties and antitumor activities. Our data demonstrate the potential of AZD3759 for the treatment of BM and LM and support its further clinical evaluation in larger trials.Â
Â
We have betaine hydrochloride,Calcium acetate,Dihydromyricetin,Andrographolide,9-methoxypsoralen,Meso-Erythritol,Magnesium lactate,Potassium Sorbate,Dihydromyricetin.
Model NO.: AZD-3759
Trademark: pharmade
Specification: 99%
Origin: China
Model NO.: AZD-3759
Trademark: pharmade
Specification: 99%
Origin: China
AZD-3759 Raw Powder Purity 99% oral CNS-penetrant EGFR inhibitorÂ