Release date: 2014-03-28 According to the US health information website mHealthNews, the US medical technology companies Sensiotec and EarlySense have developed non-contact sensor products, which do not need to be in direct contact with the patient, placed under the patient's bed or under the cushion, to achieve the disease. Tracking. Sensiotec defines this "Virtual Medical Assistant" (VMA) technology as a "true remote, contactless solution" that uses Ultra-Wideband (UWB) wireless technology developed by the US military. To capture signs of patient activity. According to the company's executives, UWB does not produce ionizing radiation, can pass through solid objects and body tissue, consumes very little power and adapts to narrow-band systems. Robert Arkin, founder and CEO of Sensiotec, said that VMA products fill the gaps that other mobile sensors can't do - it can get their disease data without touching the patient's body. He said that wearable products that come into contact with the body may sometimes be taken from the patient or malfunction due to physical activity of the patient. In addition, Sensiotec's VMA products do not require any patient operations to collect relevant data. Alkin revealed that Sensiotec products are currently used in hospital or home care settings, primarily for chronic patients with limited mobility or activity restrictions. He also said he hopes the product will be approved by the US Food and Drug Administration (FDA) for placement in the home health market. In addition to Sensiotec, another medical device company in the United States, EarlySense, has also developed a sensor that can be placed under the mattress. The Harvard Medical School has recently tested this product for 7,643 patients. Test results show that these sensors can “substantially reduce†the length of hospital stay, the number of first aid, and the length of stay in the intensive care unit (ICU) after surgery. EarlySense first demonstrated its sensor technology at the Newton-Wellesley Hospital in Massachusetts last October. Although the hospital initially was skeptical about the effectiveness of EarlySense's non-contact sensors, it was tested and found to be very helpful for patient care. Tim O'Malley, president of EarlySense, said the product is particularly well-suited for home care environments. Sensiotec's Alkin said that it will further expand the scope of application of VMA products in the future. The company has also developed an open API (application programming interface) to partner with other mobile health care providers. Sensiotec plans to include sleep apnea syndrome (SAS) and asthma in research. (Author: Zhong Tao) Source: Entropy Lithium Carbonate CAS No.554-13-2 Lithium Carbonate Basic Information
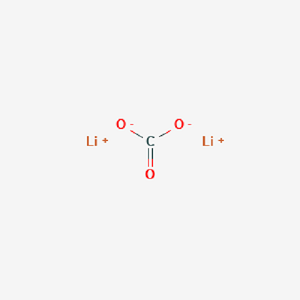
Lithium Carbonate Structure
Lithium Carbonate,Lithium Carbonate Dosage,Uses For Lithium Carbonate,Lithium Carbonate Uses,Lithium Carbonate Side Effects ShanDong YingLang Chemical Co.,LTD , https://www.sdylhgtrade.com
CAS: 554-13-2
MF: CLi2O3
MW: 73.89
EINECS: 209-062-5
Mol File: 554-13-2.mol
Melting point 720 °C
Boiling point 1342 °C(lit.)
density 2.11 g/mL at 25 °C
Fp 1310°C
storage temp. Store at +5°C to +30°C.
solubility 13g/l
form wire
Specific Gravity 2.11
color White
PH 10-11 (5g/l, H2O, 20℃)
Water Solubility 13 g/L (20 ºC)